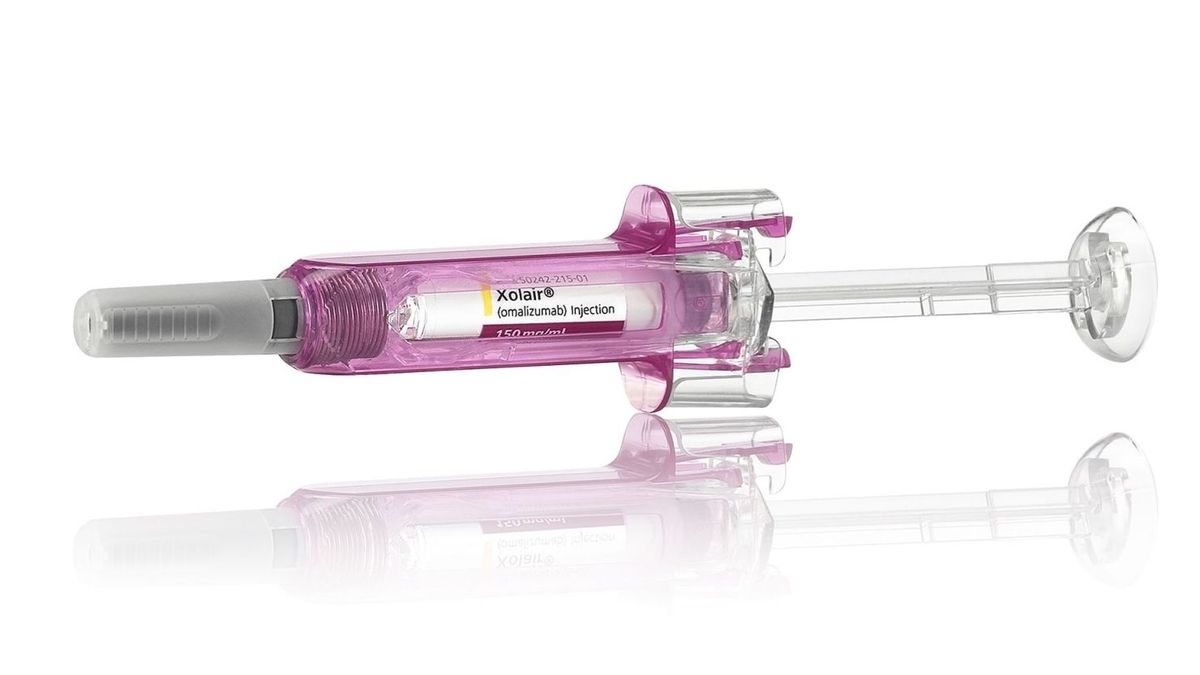


The U.S. Food and Drug Administration (FDA) has approved the use of the monoclonal antibody omalizumab, marketed as Xolair, for the reduction of allergic reactions, including anaphylaxis, caused by accidental exposure to one or more foods in adults and children aged 1 year and older with food allergies [ed1f92c7]. Xolair is a monoclonal antibody that binds to immunoglobulin E (IgE), a type of antibody that plays a crucial role in allergic responses. According to data from 2021, almost 6% of people in the United States have a food allergy, and there is currently no cure for food allergies [022ca456]. The approval of Xolair is based on data from a Phase 3 clinical trial called Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen OIT in Food Allergic Children and Adults (OUtMATCH) [ed1f92c7]. The most common side effects of Xolair observed in the trial included injection site reactions and fever [ed1f92c7]. The list price for Xolair ranges from about $2,900 to $5,000 a month [ed1f92c7].
This approval represents a significant milestone in allergy treatment and offers hope for millions of Americans with food allergies. Food allergies can be life-threatening, and the availability of Xolair as a treatment option can potentially reduce the severity of allergic reactions and improve the quality of life for those affected [022ca456].
The New England Journal of Medicine has published Phase III data from the NIH-sponsored OUtMATCH study, which showed that treatment with Xolair significantly increased the amount of peanuts, tree nuts, egg, milk, and wheat that people with food allergies could consume without experiencing allergic reactions. The study included 180 patients aged 1 to 55 years old who were unable to tolerate certain food proteins. The results demonstrated that Xolair reduced the occurrence of moderate to severe allergic reactions across multiple foods [d52d6e96].
A recent study led by Stanford Medicine has found that omalizumab, an existing allergy medication, can significantly reduce the risk of allergic reactions in children with multiple food allergies. The study, published in The New England Journal of Medicine, showed that nearly 67% of participants treated with omalizumab could safely ingest at least 600 mg of peanut protein, marking a significant leap from the less than 7% of placebo recipients who could achieve a similar tolerance. Omalizumab works by targeting and neutralizing immunoglobulin E (IgE), preventing it from activating immune cells responsible for allergic reactions [e0ba554e]. The findings of the study highlight the need for continuous exploration in the field of allergy treatments and personalized treatment plans. While the discovery offers hope for families dealing with food allergies, further research is needed to understand its long-term efficacy and safety [e0ba554e].
In the midst of this medical breakthrough, there is a controversy over higher education funding in the United States. Governor Ned Lamont is facing protests against perceived budget cuts in academia [022ca456]. The funding dispute highlights the challenges of public investment in education and the importance of adequate resources for universities and colleges to provide quality education [022ca456].
While Xolair's approval brings hope to individuals with food allergies, the education funding controversy underscores the need for balanced allocation of resources to address critical issues in healthcare and education [022ca456].