

Researchers at the Perelman School of Medicine at the University of Pennsylvania and the CMC VA Medical Center have developed a biologic 'patch' that can fix herniated discs in the back. The 'tension-activated repair patches' (TARPs) release an anti-inflammatory molecule called anakinra over time, helping discs regain tension and prevent further degeneration. The patch is activated by the body's natural motion and strengthens over time. The treatment could potentially prevent worsening pain related to disc degeneration and change the course of disease progression. The research builds upon previous work in bio-synthetic discs and mechanically-activated drug delivery systems. The study was funded by the Department of Veterans' Affairs Rehabilitation Research and Development Service and the National Institutes of Health.
Grégoire Courtine, Jocelyne Bloch, and their research team have developed therapies for bypassing and treating spinal cord injuries. They have used flexible electrodes implanted on the spinal cords of patients to relay brain signals and restore movement. The team has successfully treated patients with Parkinson's disease and paraplegia, allowing them to walk again. The research also explores the regeneration of nerve fibers through gene therapies and rehabilitation procedures. The team aims to develop therapeutic approaches that can improve the lives of many individuals with spinal cord injuries.
UNC-Chapel Hill scientists have developed a new drug delivery system called the Spatiotemporal On-Demand Patch, which can receive wireless commands from a smartphone or computer to schedule and trigger the release of drugs from individual microneedles. The patch, resembling a Band-Aid, was designed to enhance user comfort and convenience for chronically ill patients. The research team tested the patch in a mouse model using melatonin in the microneedles to improve sleep. The patch has the potential to deliver on-demand treatments for neurodegenerative disorders, such as Alzheimer's disease. It enables highly localized treatment of specific tissues or organs within the body, with drug release triggered within 30 seconds in response to an electrical signal. The patch's ability to enable joint delivery of multiple drugs could address various aspects of Alzheimer's disease. The research was funded by the UNC School of Medicine, UNC Health, the National Science Foundation, and the National Institutes of Health.
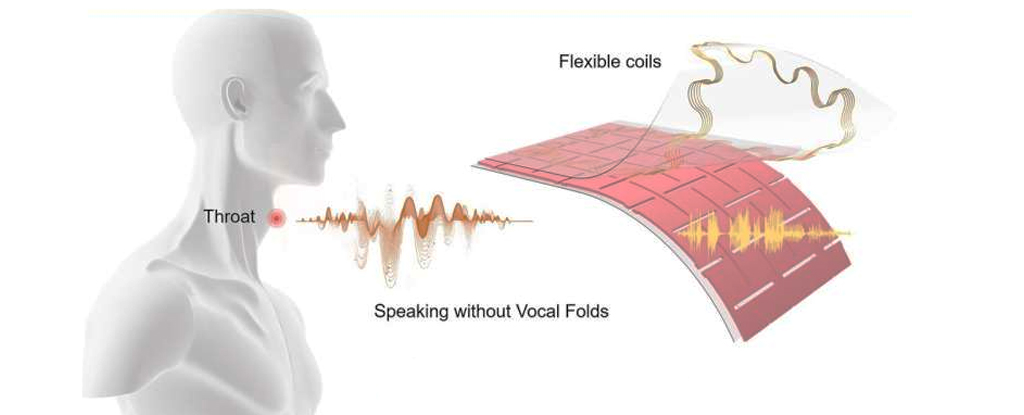
A new adhesive patch has been developed that could help people with voice disorders speak again. The patch is attached to the skin and converts the movements of the larynx muscles into electrical signals, which are then translated into speech by an AI algorithm. The patch works without needing to pick up on the vibrations of a person's vocal cords, making it useful for people with damaged vocal cords. The patch is roughly the size of a large coin and weighs just seven grams. In testing, the device accurately predicted what volunteers were saying roughly 95 percent of the time. However, the prototype is still years away from potentially being available to patients, and currently, all the sentences that can be played by the device have to be pre-recorded. [d6e9c81e]
Regenerative medicine company Orthocell Ltd has completed the first stage of its pivotal US market authorization study for its lead asset Remplir™, paving the way for global expansion and US market clearance in early 2025. The study, led by Professor Bill Walsh, assessed Remplir's safety and effectiveness in a rat sciatic nerve injury model. All nerve repair surgeries were performed without any adverse events. Orthocell plans to submit its market authorization application to the US FDA in Q4 2024 and expects to begin sales shortly after. [38ff257d]
Remedi is an organization created through a merger between Catalan Regenera Activa Worldwilde and Italian Riginera HBW. They aim to democratize treatments and expand globally. They plan to enter the Chinese and US markets, with operations starting in China next year and entry into the US expected by 2026 pending FDA approval. Remedi specializes in treating musculoskeletal pathologies using minimally invasive procedures. They have collaborations with NATO and the European Space Agency. The combined turnover of the two firms is 13 million euros, with projections to reach fifteen million this year and increase by 50% upon entering the Chinese market.