










Scientists in North Wollongong, part of a consortium called BIENCO, are on the verge of creating the world's first bioengineered cornea using 3D printing technology. The team of scientists has received a $35 million grant to develop the machinery and manufacturing line necessary to print and reproduce corneas on a large scale. The goal of this groundbreaking project is to address the shortage of donated corneas and restore sight to millions of people. The first cornea transplants using bioengineered corneas are expected to take place within the next few years. In addition to its impact on sight restoration, the project also has wider implications for the development of other synthetic body parts and sets up a manufacturing pathway for bioengineered products. The consortium includes experts from the University of Sydney, the University of Melbourne, Queensland University of Technology, the Centre for Eye Research Australia, and the NSW Organ & Tissue Donation Service, with Professor Gerard Sutton from the University of Sydney serving as the medical lead. The $35 million grant awarded to BIENCO is the largest in Australia's history for eye research [b2c768da].
The development of a bioengineered cornea using 3D printing technology represents a significant medical breakthrough. The shortage of donated corneas has long been a challenge in the field of ophthalmology, and the ability to manufacture corneas using 3D printing could revolutionize the treatment of corneal diseases and injuries. By utilizing 3D printing technology, scientists can create corneas that are tailored to each patient's specific needs, improving the chances of successful transplantation and reducing the risk of rejection. This breakthrough has the potential to restore sight to millions of people worldwide and significantly impact the field of regenerative medicine [b2c768da].
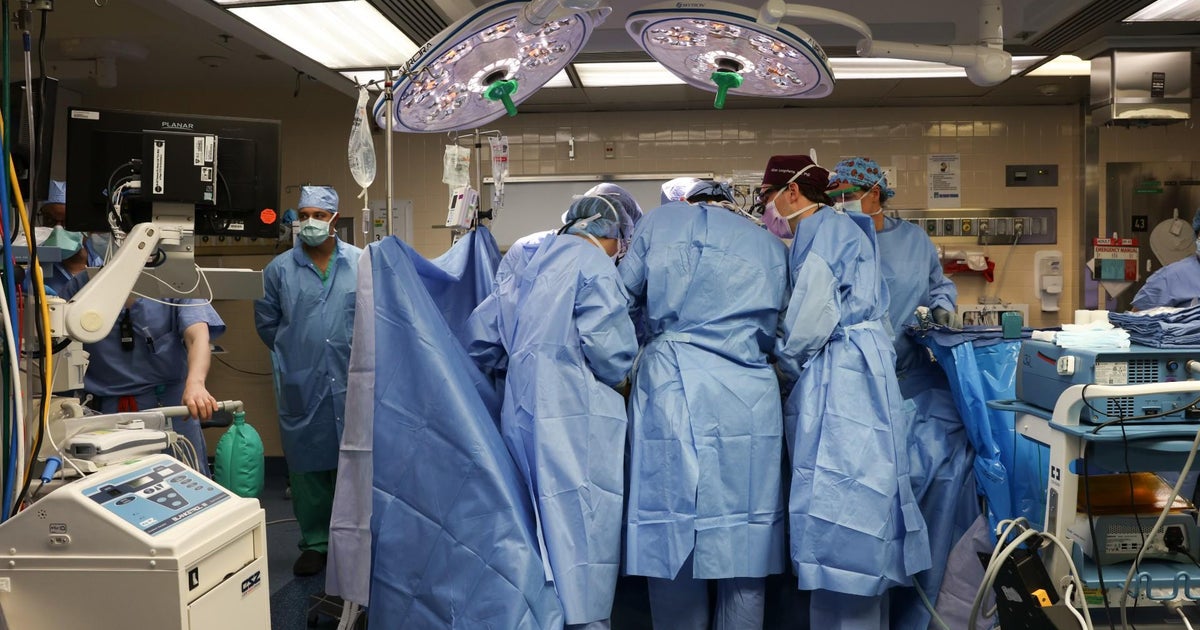
In another groundbreaking milestone, surgeons at Massachusetts General Hospital in Boston have performed the first transplant of a genetically modified pig kidney into a living human. The four-hour operation was carried out on a 62-year-old man suffering from end-stage kidney disease. The patient, Richard Slayman, who is Black, had received a transplant of a human kidney in 2018 but it began to fail five years later. The pig kidney transplant is seen as a major milestone in providing more readily available organs to patients and could offer hope to the thousands of people who need a transplant to survive. The procedure has the potential to address the chronic problem of organ shortages worldwide, particularly among ethnic minorities who suffer from high rates of kidney disease. The pig kidney used for the transplant had been genetically edited to remove harmful pig genes and add certain human genes. This is the first time a pig kidney has been transplanted into a living person, although pig kidneys had been transplanted into brain-dead patients before [2c9d424f] [9aa369b5] [e097540d].
The successful transplantation of a genetically modified pig kidney into a living human represents a significant advancement in the field of xenotransplantation. Previous studies have shown success in transplanting animal organs into human patients, but this is the first time a genetically edited pig kidney has been used. The procedure offers hope for addressing the shortage of organs available for transplantation, as there are currently over 103,000 people on the national transplant waiting list in the US. However, questions remain about the long-term efficacy, safety, and ethics of using edited animal organs for transplantation. The field of xenotransplantation raises concerns about the possibility of spreading animal viruses to humans and the ethical implications of using animal organs in people. Nonetheless, this transplant represents a potential breakthrough in solving the problem of unequal access to kidney transplants for ethnic minority patients due to the organ shortage and other barriers [ca5859f5].
A 62-year-old man from Weymouth, Massachusetts, received the world's first successful transplant of a genetically edited pig kidney at Massachusetts General Hospital. The pig kidney, which had 69 genomic edits, was transplanted into the patient who was living with end-stage kidney disease. The surgery took approximately four hours and is a major milestone in providing more readily available organs to patients. The patient, Richard Slayman, had previously received a kidney transplant from a deceased human donor in December 2018 but experienced kidney failure five years later and resumed dialysis. Slayman's nephrologist and the Transplant Center team suggested a pig kidney transplant, which he saw as a way to provide hope for others in need of a transplant. The pig kidney was provided by eGenesis of Cambridge, Massachusetts, and was genetically edited to remove harmful pig genes and add certain human genes to improve compatibility. The procedure was performed under a single FDA Expanded Access Protocol, known as compassionate use, to gain access to experimental treatments when no comparable options exist [dffa8efe] [3eee6296] [555f301a].
Mass General Hospital in Boston has successfully transplanted a genetically edited pig kidney into a 62-year-old man with end-stage kidney disease. The pig kidney, which was genetically edited using CRISPR-Cas9 technology, was provided by eGenesis of Cambridge. The recipient of the pig kidney, Richard Slayman, is a Black man, which is significant as African Americans have higher rates of deadly kidney disease. The success of this pioneering surgery opens a new frontier in organ transplantation and provides hope for countless individuals suffering from end-stage renal disease. Over 100,000 people in the U.S. await an organ for transplant, and 17 people die each day waiting for an organ. End-stage kidney disease rates are estimated to increase 29-68 percent in the U.S. by 2030 [1460fe97].
Data from the International Society for Heart and Lung Transplantation (ISHLT) indicates that lung transplantation can be performed in advanced age candidates and provides life-extending benefits. Despite age over 65 being characterized as a relative contraindication, there has been a steady rise in the age threshold considered for lung transplantation. A retrospective cohort study conducted by Jingyu Chen et al. at Wuxi Lung Transplant Center found that lung transplantation can be performed in candidates aged 65 and above, with recipients aged 70 and above having more frequent comorbidities. The study also suggests that bilateral transplantation is associated with improved long-term survival, while single lung transplantation offers a survival benefit within the first year post-transplant [8dc00d46].
A mother shares her gratitude for the organ donor who gave her three more years with her daughter. The mother's daughter was diagnosed with a rare liver disease at the age of 2 and was in need of a liver transplant. After being on the waiting list for a year, a suitable liver became available and the transplant was successfully performed. The mother expresses her appreciation for the selfless act of the organ donor, as it allowed her daughter to live a fulfilling life for three more years. The article highlights the importance of organ donation and the impact it can have on the lives of those in need [269f801c].
Richard 'Rick' Slayman, the first recipient of a genetically modified pig kidney transplant, has died nearly two months after the procedure at Massachusetts General Hospital. The transplant team expressed their condolences and stated that they did not have any indication that his death was a result of the transplant. Slayman had previously undergone a kidney transplant in 2018 but had to go back on dialysis when it showed signs of failure. His family thanked the doctors for their efforts and stated that Slayman's hope and optimism will endure forever. Xenotransplantation, the use of animal organs for human patients, has been challenging due to the human immune system's rejection of foreign animal tissue. However, recent attempts with genetically modified pigs have shown promise. Over 100,000 people are on the national waiting list for a transplant, with most of them being kidney patients [9f45a63b].
Kidney disease is a leading cause of death in the U.S., with Black Americans three times more likely than white Americans to develop kidney failure. The U.S. organ transplantation system rates donor kidneys using the kidney donor profile index, which includes race as a factor. However, race is a poor indicator of genetic diversity, and the observed differences in transplantation outcomes may be due to genetics rather than race. Reducing the number of wasted kidneys and making organ transplantation more equitable could be achieved by using genetic factors instead of race when evaluating donor kidneys. Identifying the factors that lead to some kidneys donated by Black people to fail at higher rates is also important. This flawed system raises ethical concerns about justice, fairness, and stewardship of a scarce resource [d45393d9].